Clinical trial planning
Clinical trial supply planning and management
Planning and management of the clinical supply chain is not just an operational task – it is a strategic discipline that ensures patients receive their medication on time and trials progress without disruption. At KLIFO, we combine deep expertise with end-to-end execution to design supply plans that are resilient, cost-efficient, and tailored to the realities of modern drug development.

Why a robust supply plan matters
The supply plan is the backbone of every clinical trial. It connects patient recruitment with production, packaging, labelling, storage, and distribution. Done right, it prevents costly shortages, avoids unnecessary overage, and ensures availability of trial medicines across global markets.
Key goals of a strong clinical supply plan:
- Accurate forecasting – anticipating trial demand and aligning supply with enrolment curves.
- Operational execution – ensuring finished product is where it needs to be, when it is needed.
- Risk resilience – identifying potential bottlenecks early and building mitigation strategies.
Patient focus – making sure participants never miss a dose, regardless of geography.

Demand planning
Drug availability (IMP and comparator), unpredictable recruitment, drug expiry dates and protocol amendments need to be considered when designing clinical supply processes. KLIFO’s Clinical Supply Managers work closely with our clients to develop the best strategy for their protocol.
Our plans consider:
- Campaign-based vs just-in-time packaging strategies.
- Procurement strategies for comparator products, balancing anticipated need with product availability and shelf life.
- Recommended buffer stock levels to ensure availability without excessive waste.
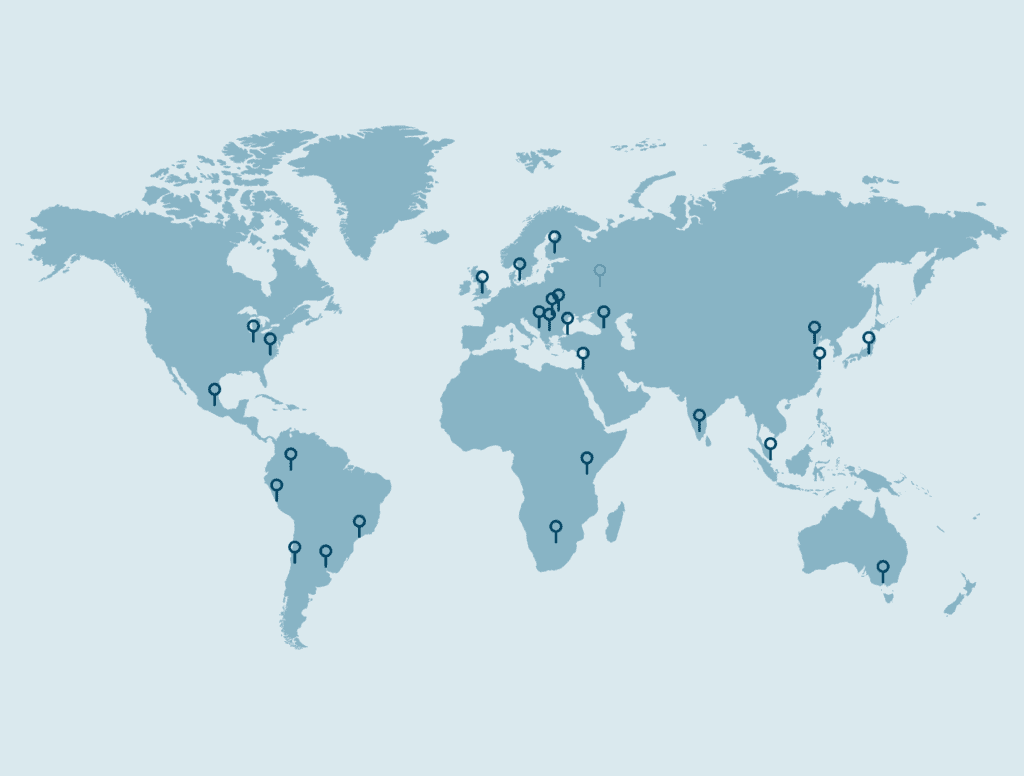
- Optimum global distribution strategies that balance cost, availability of product, target patient population (e.g. rare diseases vs widespread conditions) and anticipated number of shipments per country or region.
- Plans should also consider factors such as trial designs – for example, strategies for adaptive and decentralised trials may differ from traditional trial designs.

Risk management and continuity
Every trial faces uncertainties – from protocol changes to global comparator shortages. At KLIFO, we apply proactive and reactive risk management frameworks to safeguard supply continuity.
Our strategies include:
- Back up providers for critical materials (e.g. comparator drugs) and services (e.g. in-country depots).
- Just-in-time packaging and distribution strategies to support product pooling, ensuring utilisation of available inventory is maximised.
- KLIFO facility has full back-up power supply – storage of refrigerated materials can be divided between separate large cold rooms to minimise product risk in unlikely event of refrigerator failure.
- Contingency planning for site-level resupply, including flexible processes to rapidly respond to any emergency scenario.
This ensures that, even when disruptions occur, patient safety and trial integrity are never compromised.

Clinical trial planning
Site resupply and logistics excellence
Timely distribution to clinical sites is where supply planning meets execution. KLIFO operates purpose-built packaging suites and extensive storage capacity, combined with global depot and distribution networks.
We manage:
- Packaging and labelling of IMPs, comparators, and ancillaries.
- Global trade compliance and import/export services.
- Direct-to-site shipments from KLIFO, and through proactive management of our approved depot partners.
- Service level agreements (SLAs) between KLIFO and our partners, and ongoing KPI monitoring of our own, and our partners’, performance ensures your trial remains uninterrupted.

Clinical trial planning
Balancing cost, efficiency, and sustainability
Supply planning is not only about security – it’s about optimising resources. We help sponsors strike the right balance between cost and waste, without compromising timelines.
Our approach includes:
- Budget planning with transparent cost tracking.
- Packaging and distribution strategies to help minimise overage and potentially wasted drug product.
- Energy-efficient facilities and reusable shipping solutions help minimise the environmental impact of the clinical supply chain and aligns with our own, and our clients’, ESG goals.
Guidance through every phase
KLIFO supports clinical trial from first-in-human through to large scale global phase 3 and post-marketing studies. As trial grow, so too does the scale and scope of services required to ensure successful execution.
In early clinical development, speed and flexibility are often priorities for our clients. While these remain important throughout clinical development, our global knowledge and experience, capacity and global distribution network are highly valued.


Why choose KLIFO as your supply planning partner?
At KLIFO, supply planning is more than numbers on a spreadsheet. It is guided by senior experts who understand both strategy and operations – ensuring nothing is lost between planning and execution.
Partnering with KLIFO means:
- 30+ years of CTS expertise across biotech, pharma, and CRO-led studies.
- Vertical insight – from protocol design to global distribution.
- Embedded collaboration – working as part of your team, not just beside it.
- Agility and flexibility – rapid adaptation to trial changes.
End-to-end accountability – one partner from planning through delivery.
What is the typical background of your Project/Clinical Supply Managers (CSMs)?
Most of the senior members of our CSM team join us with more than a decade’s experience in CTS. In many cases, this is based on experience at Sponsor companies. We feel this is a big differentiator for KLIFO. Our CSMs have sat where our clients sit. They understand the challenges companies face when outsourcing CTS projects. They know how stressful it can be if communication ‘goes dark’, and understand that early communication of potential risks to timelines allows clients time to mitigate against these.
For its population size, Denmark ‘punches above its weight’ in terms of investment in pharmaceutical and biotech companies. As a result, we are fortunate to have an experienced workforce available locally.
However, a vibrant local industry also creates competition for employees. KLIFO has proactively invested in a Graduate program to train the next generation of CSMs. This is a 9 month program, during which we train recent life sciences graduates to develop expertise in CTS. Graduates are able to ‘learn from the best’ through an intensive combination of classroom and on-the-job training. Graduates from the program are offered permanent positions in KLIFO’s CSM team, where they have the opportunity to continue to develop their CTS experience.
How does KLIFO help us manage risks in the clinical supply chain?
This comes down to experience. While many of our clients have managed investigational products from discovery to commercialization, others come to KLIFO having never developed a clinical supply strategy before. One of the advantages of having such an experienced team is that if something can go wrong, someone in the KLIFO team has probably directly experienced the same issue and had to develop a solution. This allows us to work with clients to develop a checklist of potential risks specific their project so we can jointly develop contingency plans. These have to be collaborative – for example, if risks relate to a process not controlled by KLIFO, we can develop a contingency plans to react to the risk but may not be able to eliminate the risk without involving the client.
Is KLIFO able to offer Clinical Supply Management as a standalone service?
The honest answer to this is…it depends on other commitments at the time of asking. Several of our early phase clients lack dedicated CTS resources internally, and ask if we can fulfill this role for them. If we have resources available, we are willing to do this – we have even had cases where we have project managed competitors to ensure they deliver according to the client’s plan. However, this is unusual and most of our CSM resources are focused on projects in which KLIFO is delivering services such as packaging and distribution.
Clinical Supplies is an area where we see significant deviation from budget. How can you help us prevent this?
This is not an unusual comment. CTS budgets are usually set long before a trial starts, and often involve making a lot of assumptions over how a trial will run. Then the trial starts and reality takes over! This is a problem for Distribution in particular.
If this is a concern and is requested before the trial starts, KLIFO can set up processes to track spending against budget by service area. If budget begins to deviate from expected levels, KLIFO can see if there is a reason for this, and recommend changes that may help get spending back on track. For example, if there are significantly more shipments than expected, is this due to how the IRT/RTSM system was set up?